Diabetes Deep Dive: Science, Breakthroughs, and the Path Forward

Diabetes continues to rise at an alarming rate, both nationally and globally, with significant negative impacts on both healthspan and lifespan. This growing prevalence is largely due to a combination of modifiable lifestyle factors including increased caloric intake and reduced physical activity. Over time, these patterns compound and further contribute not only to impaired glucose regulation but also to downstream conditions such as increased body mass index (BMI), further increasing the risk of cardiovascular disease, inflammation, metabolic decline and ultimately metabolic syndrome.
In this article, I will examine the evolving scientific understanding of diabetes, including its pathophysiology, diagnostic markers and evidence based treatment strategies. From prevention to reversal, I will explore actionable lifestyle interventions supported by clinical data, as well as the role of emerging tools such as real time digital feedback, novel pharmacological agents and innovative health focused wearables helping reshape diabetes care toward a healthier future.
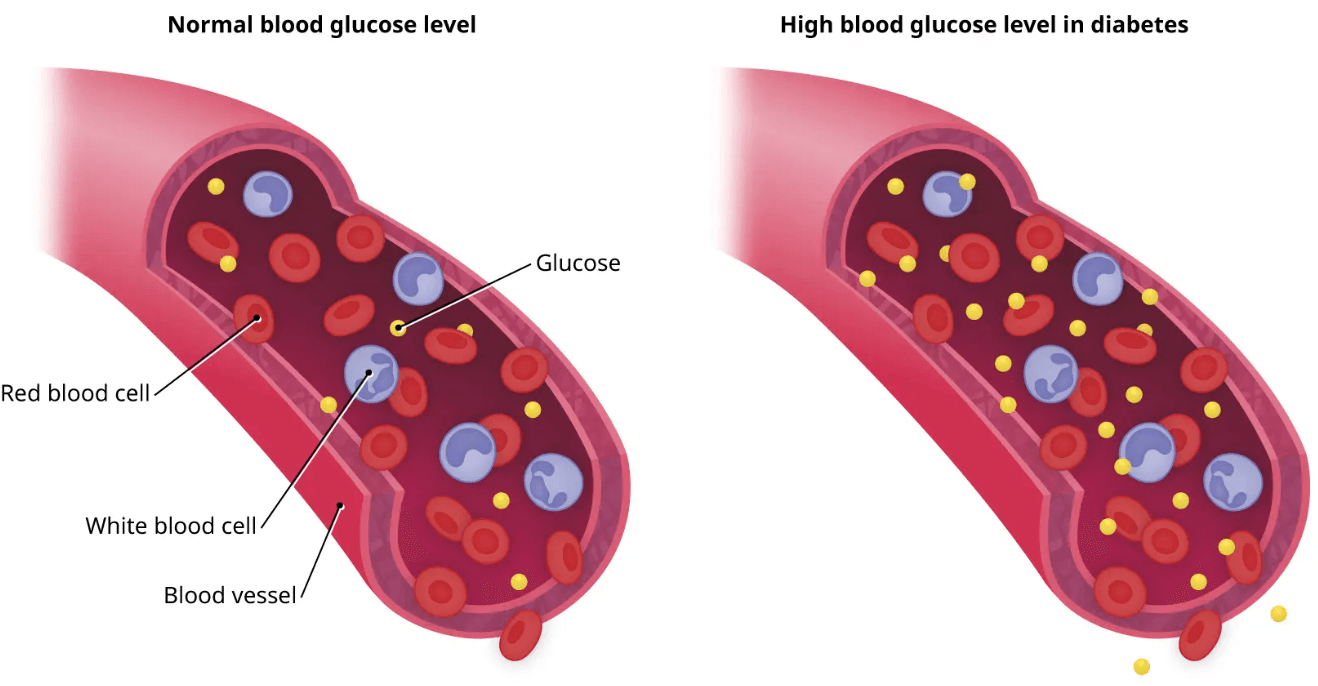
Hyperglycemia, or elevated blood glucose, poses serious health risks due to its compounding negative impact on the body. Hyperglycemia damages blood vessels through the process of glycation, where sugars bind to proteins, forming advanced glycation end-products (AGEs) that damage blood vessels and promote atherosclerosis, hypertension and impaired circulation. Eventually, the pancreas compensates by increasing insulin secretion, which over time contributes to beta-cell strain and dysfunction (a key driver of Type 2 diabetes). High glucose levels also impair nerve function, resulting in diabetic neuropathy, and can damage the small vessels in the kidneys and eyes, increasing the risk of both kidney failure and vision loss. Immune function is also suppressed in hyperglycemic states, making the body more susceptible to infections and delayed healing. High glucose levels also activate inflammatory pathways, fueling systemic low grade inflammation, which is involved in the development of cardiovascular disease, Alzheimer’s disease and certain cancers. Needless to say, maintaining healthy blood glucose levels is essential not only for preventing diabetes but for supporting overall metabolic and physiological health.
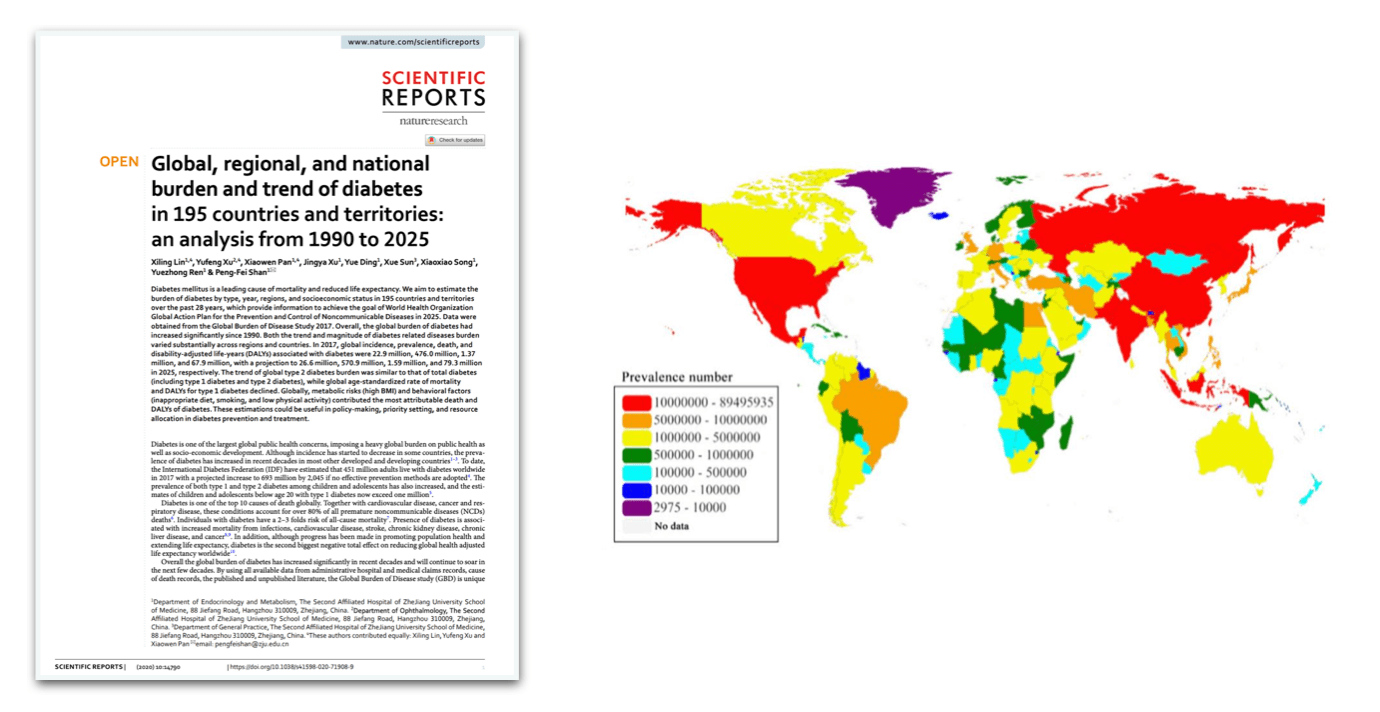
Globally, diabetes affects over 537 million adults (aged 20–79), according to the International Diabetes Federation (IDF). Alarmingly, this figure is expected to rise to 783 million by 2045. Of those currently living with diabetes, approximately 90–95% have Type 2 diabetes, a condition driven largely by lifestyle related factors such as poor diet, sedentary behavior and rising obesity rates. Type 1 diabetes, an autoimmune form typically diagnosed in childhood or early adulthood, accounts for about 5–10% of cases worldwide. Aside from the well established Type 1 and Type 2 diabetes, an estimated 541 million adults have prediabetes. An impaired glucose regulation condition, prediabetes significantly increases the risk of progressing to Type 2 diabetes. Leading health bodies such as the WHO, the CDC, and the IDF, emphasize the rising burden of diabetes as one of the most pressing global health challenges. Importantly, prevalence is growing most rapidly in low socioeconomic areas with the global cost of diabetes care estimated at over $966 billion USD per year. Effective prevention, management, and reversal strategies are essential to addressing this escalating health, economic, and societal burden.
Prediabetes is defined by elevated blood glucose levels (fasting glucose between 100 - 125 mg/dL and an HbA1c ranging from 5.7% to 6.4%) resulting from reduced insulin sensitivity and early beta-cell dysfunction. This metabolic imbalance impairs efficient glucose uptake by peripheral tissues while simultaneously increasing hepatic glucose production, contributing to chronic hyperglycemia. Without timely intervention, prediabetes frequently progresses to Type 2 diabetes. Lifestyle modifications, including diet and regular exercise, aim to enhance insulin sensitivity and preserve beta-cell function. When necessary, later discussed supplemental or pharmacological treatments may be utilized to delay or prevent disease onset.
Hepatic glucose production (HGP) is the liver's process of producing and releasing glucose into the bloodstream, primarily during fasting or between meals to maintain stable blood glucose levels. It occurs through two main mechanisms; glycogenolysis, the breakdown of stored liver glycogen, and gluconeogenesis, the synthesis of glucose from non-carbohydrate sources like lactate, glycerol and amino acids. While HGP is tightly regulated by insulin in healthy individuals, this control is impaired in insulin resistance and Type 2 diabetes, leading to excessive glucose output by the liver and contributing to elevated fasting blood glucose levels only to further exacerbate the fundamental issue.

Extensive research has firmly established that early intervention in individuals with prediabetes can significantly delay or prevent the progression to Type 2 diabetes. The Diabetes Prevention Program (DPP) demonstrated that intensive lifestyle changes (weight loss, dietary improvements, and at least 150 minutes of weekly exercise) reduced diabetes incidence by 58%, outperforming the pharmacological agent metformin. Similarly, the long-term Da Qing Study confirmed that lifestyle interventions targeting diet and exercise lowered the risk of developing diabetes by 26–35% and yielded additional health benefits over decades of follow up. These studies, in additional to numerous real world anecdotes in the recreational and gym setting, provide compelling evidence that structured lifestyle interventions are not only highly effective for managing prediabetes but also critical for reducing long term health risks.
Type 1 diabetes is an autoimmune condition in which the immune system attacks and destroys pancreatic beta cells, resulting in an absolute deficiency of insulin. This deficiency impairs glucose uptake by tissues, causing elevated blood glucose levels, and without insulin replacement, chronic hyperglycemia can lead to both acute and long-term complications. Effective management requires lifelong exogenous insulin therapy, careful glucose monitoring, and lifestyle strategies to maintain metabolic control and reduce complications. Early diagnosis and tight glucose regulation are critical to preserving health and preventing diabetic complications.
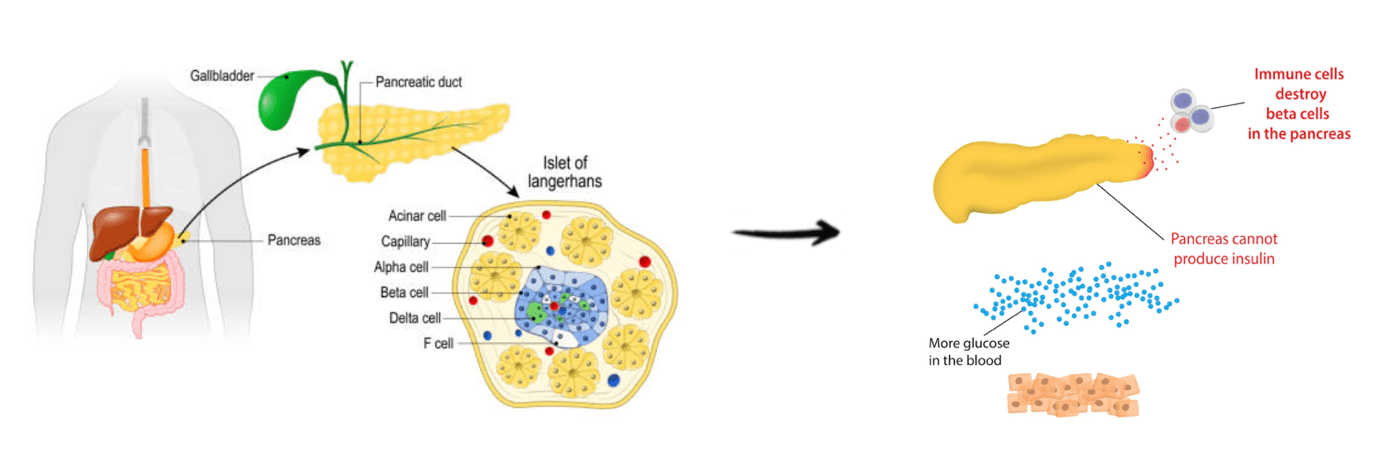
While insulin therapy is essential for survival, strategic lifestyle modifications can enhance insulin efficiency and reduce the total daily dose required, improving both glycemic control and long term outcomes. For example, a study examining a low carbohydrate diet (<100 g/day) in individuals with Type 1 diabetes found reduced time spent in hypoglycemia, decreased glycemic variability, and modest weight loss compared to a higher carbohydrate diet. Minimizing hypoglycemia is particularly important, as severe low blood sugar events can cause dizziness, seizures, or loss of consciousness. Stabilizing glucose fluctuations reduces physiological stress and improves overall metabolic control, while modest weight loss enhances insulin sensitivity and cardiovascular health, further supporting long term disease management.

Given the loss of endogenous insulin production, individuals with Type 1 diabetes rely on insulin injections or pumps to regulate blood glucose. Inconsistencies between insulin dosing, nutrient intake, and physical activity can precipitate hypoglycemia, often more frequent than hyperglycemic events, especially during exercise, overnight or with missed meals. Continuous glucose monitors (CGMs) are especially important in these scenarios, providing real time feedback that allows timely intervention and reduces the risk of severe hypoglycemia. On average, healthy adults produce approximately 35 units of insulin per day, though this varies between individuals and fluctuates throughout the day. Basal-bolus therapy, delivered via injections or pumps, combines long acting basal insulin to control fasting glucose with rapid acting bolus insulin to manage postprandial spikes, which is the current standard for approximating natural insulin dynamics.
The landmark Diabetes Control and Complications Trial (DCCT) demonstrated that intensive glucose control (maintaining blood glucose as close to normal as safely possible) significantly reduces the risk of microvascular complications, including retinopathy, nephropathy and neuropathy, compared to conventional therapy. The subsequent EDIC follow up revealed that these early benefits translated into long term reductions in CVD and all-cause mortality, even after differences in glucose control between groups diminished, known as “metabolic memory.” Together, these findings highlight the critical importance of early, sustained and tightly managed glucose control in Type 1 diabetes. By combining precise insulin therapy, lifestyle interventions, and modern monitoring technologies like CGMs, individuals can substantially improve both short and long term health outcomes, providing a strong foundation for managing this lifelong condition.
Type 2 diabetes (T2D) is a chronic metabolic disorder characterized by insulin resistance, where the body’s cells do not respond effectively to insulin, leading to a progressive decline in pancreatic beta-cell function. This dual defect leads to elevated blood glucose levels (hyperglycemia). Dr. Gerald Shulman’s research indicates that a substantial portion of U.S. adults, between 25 - 50%, exhibit insulin resistance without obvious symptoms. Often described as a “silent” or subclinical metabolic dysfunction, this condition frequently goes undiagnosed until more advanced stages. Critically, this further stresses the importance of early detection and action. Using novel techniques such as magnetic resonance spectroscopy, Shulman demonstrated that insulin resistance often begins in skeletal muscle, where impaired glucose uptake and intracellular lipid accumulation disrupt normal metabolic function. These findings align with research showing skeletal muscle as a primary site of disease onset, setting the stage for subsequent non-alcoholic fatty liver (NAFL), hepatic insulin resistance and ultimately the development of Type 2 diabetes. Given these findings, skeletal muscle insulin resistance represents an early and critical initiating event in the pathogenesis of the disease.
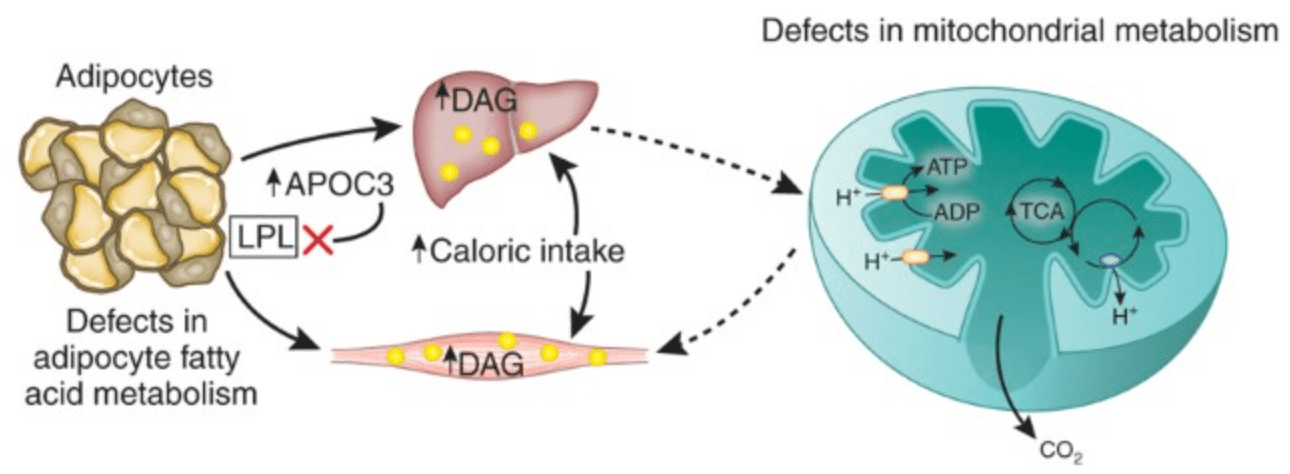
At a cellular level, excess caloric intake leads to overproduction of acetyl-CoA in mitochondria. When energy input exceeds the capacity of the TCA cycle, metabolic intermediates build up, generating mitochondrial stress and altering cellular balance. This reduces the ability of muscle cells to effectively use glucose, contributing to insulin resistance. Compounding this effect, accumulation of intracellular diacylglycerols (DAGs) activates specific enzymes in muscle and liver cells. These enzymes interfere with normal insulin signaling, limiting glucose uptake in skeletal muscle and reducing the liver’s ability to suppress glucose production. Together, these mechanisms help explain how metabolic overload drives early insulin resistance and highlights the importance of lifestyle interventions, particularly exercise and dietary strategies, to prevent progression toward Type 2 diabetes.

Dr. Gerald Shulman’s work identifies skeletal muscle insulin resistance as the earliest detectable defect in type 2 diabetes, where impaired insulin signaling and lipid accumulation reduce GLUT4 translocation and glucose uptake. This dysfunction triggers compensatory hyperinsulinemia, which initially maintains glucose homeostasis but further promotes lipid buildup in muscle and liver, reinforcing insulin resistance. Over time, β-cell compensation fails, leading to metabolic decline and Type 2 diabetes. Building on this foundation, Dr. Ralph DeFronzo broadened the perspective with his “Ominous Octet,” describing 8 interrelated defects. These include insulin resistance in muscle, liver, and adipose tissue, beta-cell dysfunction, dysregulated incretin hormones, increased hepatic glucose production, abnormal glucagon secretion, renal glucose reabsorption and central signaling abnormalities. Together, these insights reveal Type 2 diabetes as a progressive, multi-organ disorder and emphasize the need for early, multi-targeted interventions combining lifestyle, supplemental and pharmaceutical strategies. In doing so, one aims to preserve beta-cell function, improve systemic insulin sensitivity and slow disease progression
Beyond glucose control, insulin signaling also stimulates endothelial nitric oxide synthase (eNOS), promoting vascular health. When this pathway is impaired, patients remain at elevated cardiovascular risk even if blood glucose appears controlled, since reduced nitric oxide availability contributes to vascular dysfunction. Kidney glucose transport further compounds the problem. Sodium-Glucose Cotransporter 1 (SGLT1) and SGLT2 reabsorb the majority of filtered glucose, worsening hyperglycemia in insulin-resistant states. Together, impaired insulin signaling, beta-cell stress, disrupted vascular regulation and renal glucose reabsorption explain the interconnected mechanisms driving both metabolic and cardiovascular complications in diabetes.

Diagnosing and monitoring diabetes requires more than a single snapshot; rather, a combination of complementary tools to provide insight into both short and long term trends. One key diagnostic tool is the Oral Glucose Tolerance Test (OGTT), which evaluates the body’s efficiency in processing glucose. After fasting overnight for 8 to 12 hours, a baseline blood glucose level is measured. The patient then consumes a high glucose beverage containing 75 grams of sugar, and subsequent blood samples are collected at intervals (typically every 30 minutes for two hours) to observe how blood glucose levels change over time. Normal glucose levels fall below 140 mg/dL at the two-hour mark, whereas levels between 140 and 199 mg/dL suggest impaired glucose tolerance (prediabetes), and readings of 200 mg/dL or higher confirm a diagnosis of diabetes. The OGTT offers a dynamic, real time snap shot of glucose regulation and is highly sensitive for detecting early metabolic dysfunction. As Dr. Defronzo highlights, "the single best predictor of whether someone will develop diabetes or not is a one hour glucose level greater than 155 mg/dL."

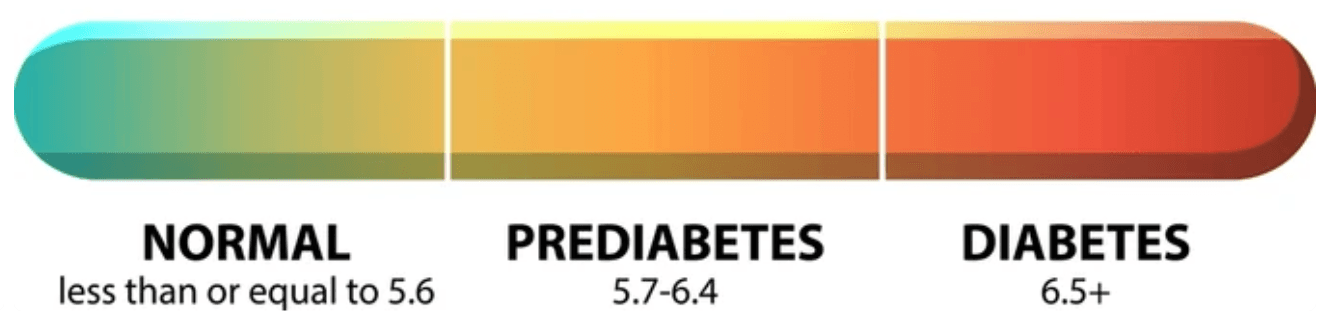
Hemoglobin A1C (HbA1c) is a blood test that reflects a person’s average blood glucose levels over the past two to three months. It works by measuring the percentage of glucose that has bonded to hemoglobin, the protein in red blood cells that carries oxygen. Since red blood cells live for about 120 days, the test provides a long term view of glucose exposure. To measure HbA1c, a small blood sample is taken and analyzed in a laboratory using standardized assays such as high-performance liquid chromatography (HPLC). The result is reported as a percentage. An HbA1c below 5.7% is considered normal, 5.7% to 6.4% indicates prediabetes, and 6.5% or higher on two separate tests confirms a diagnosis of diabetes. The HbA1c test is widely used in both diagnosis and ongoing management of diabetes due to its convenience and correlation with long term risk of diabetes related complications. Importantly, multiple factors can impact the accuracy of a HbA1c test, and it is often used a supplemental diagnostic tool, rather than a standalone measure.
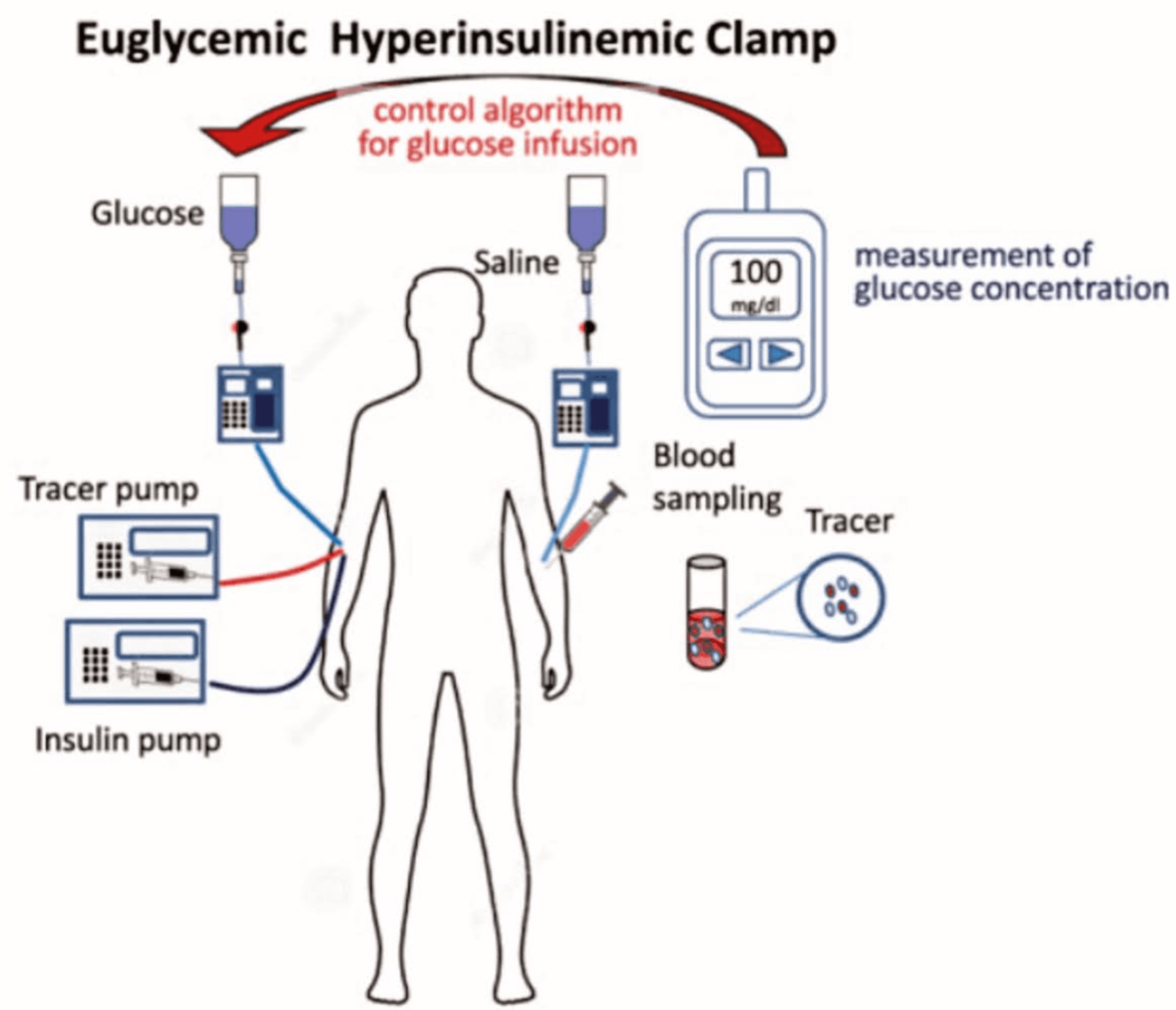
Insulin Clamping, originated by Dr. Ralph DeFronzo’s hyperinsulinemic–euglycemic clamp, is considered the gold standard for measuring insulin sensitivity, has been key in identifying skeletal muscle as the primary site of insulin resistance in Type 2 diabetes. In this method, insulin is infused at a constant high level while glucose is simultaneously administered to maintain normal blood sugar, isolating how effectively tissues respond to insulin. The amount of glucose needed to maintain euglycemia reflects insulin stimulated glucose uptake, mainly in skeletal muscle. Using this approach, DeFronzo showed that impaired muscle glucose uptake is an early and central defect in the development of Type 2 diabetes, often preceding liver insulin resistance and beta-cell dysfunction. These findings highlight why improving muscle insulin sensitivity through exercise, diet, and targeted therapies is critical for managing and preventing diabetes.
Continuous glucose monitors (CGMs), once reserved for individuals with diabetes, are now embraced by health conscious consumers seeking preventive tools that deliver real time, personalized data. By continuously tracking glucose, CGM users can observe how specific foods, meal timing, sleep quality and physical activity affect their blood glucose responses, turning objective health data into actionable feedback rooted in quantifiable metabolic trends. For example, a short post-meal walk enhances glucose uptake through GLUT4 activation in skeletal muscle, the body’s largest glucose sink. These insights not only guide but also reinforce behavior change, making CGMs powerful tools for improving insulin sensitivity, supporting long term metabolic resilience, and extending their utility far beyond traditional clinical use. To better appreciate how CGMs provide this level of actionable insight, it helps to understand how the devices measure glucose in the body.
CGMs operate by inserting a small sensor into the subcutaneous tissue, where it samples glucose levels from the interstitial fluid rather than directly from the bloodstream. Although the sensor measures glucose in interstitial fluid, numerous studies have demonstrated that interstitial glucose concentrations closely correlate with blood glucose, particularly when tracking trends over time. Despite a minimal (5-10 minutes) blood glucose lag/delay, CGMs reliably capture trends that inform real time behavioral decisions. Being a validated, valuable tool for behavioral feedback and metabolic tracking has led to their widespread acceptance for glycemic monitoring and management. Beyond simply tracking numbers, CGMs help translate glucose data into patterns that reveal how daily choices influence long term metabolic health. By making these patterns visible, CGMs empower users to adjust meals, activity and lifestyle choices in real time.
With these glucose patterns visible, their true value emerges when interpreted and applied. Among the most practically relevant insights is “time in range,” or the proportion of the day spent within healthy glucose thresholds. Beyond average glucose values, CGMs capture the dynamics of post-meal responses, including how high glucose peaks rise, how sharply they fall and how variable those swings are. Importantly, large spikes followed by steep drops have been shown to drive hunger and cravings, making it harder for one to sustain weight management. This also translates to higher circulating insulin levels, which the body uses to control glucose increases. Chronically elevated insulin suppresses lipolysis (breakdown of stored fat) and sustains hepatic glucose production, both of which impair the ability to shrink fat cells and lose weight. These patterns highlight how CGM feedback can guide everyday choices to prevent the progression of obesity and Type 2 diabetes.

Humans have undergone over thousands years of evolutionary pressure that favored the consumption of sugar. What once served as a survival mechanism during periods of food scarcity has become a modern preference, often leading to overconsumption and metabolic dysfunction. Not all carbohydrates, however, are metabolically equal. Emerging evidence reveals that the source and form of carbohydrate can significantly influence glucose regulation, appetite and long term metabolic health. Fructose, in particular, elicits unique effects and is primarily metabolized in the liver, bypassing key regulatory steps that control glucose metabolism, causing a blunted effect on satiety hormones such as GLP-1 and leptin. Excessive fructose intake, especially from added sugars or highly processed foods, can promote overconsumption, drive triglyceride accumulation in the liver, contribute to NAFLD, uric acid and worsen insulin resistance and inflammation. Unlike glucose, fructose does not significantly stimulate insulin secretion or subsequent leptin release, two hormonal signals critical for appetite and energy balance. These mechanisms collectively make chronic overconsumption of fructose a particularly disruptive factor in metabolic health. Importantly, it's the chronic overconsumption of fructose, often from added sugars like high-fructose corn syrup or sugary beverages, combined with calorie surplus, that leads to the unique metabolic harms associated with fructose. As Dr. Peter Attia mentions, "the more processed, the more liquid and the more fructose you are consuming, the more likely you will be to over consume."
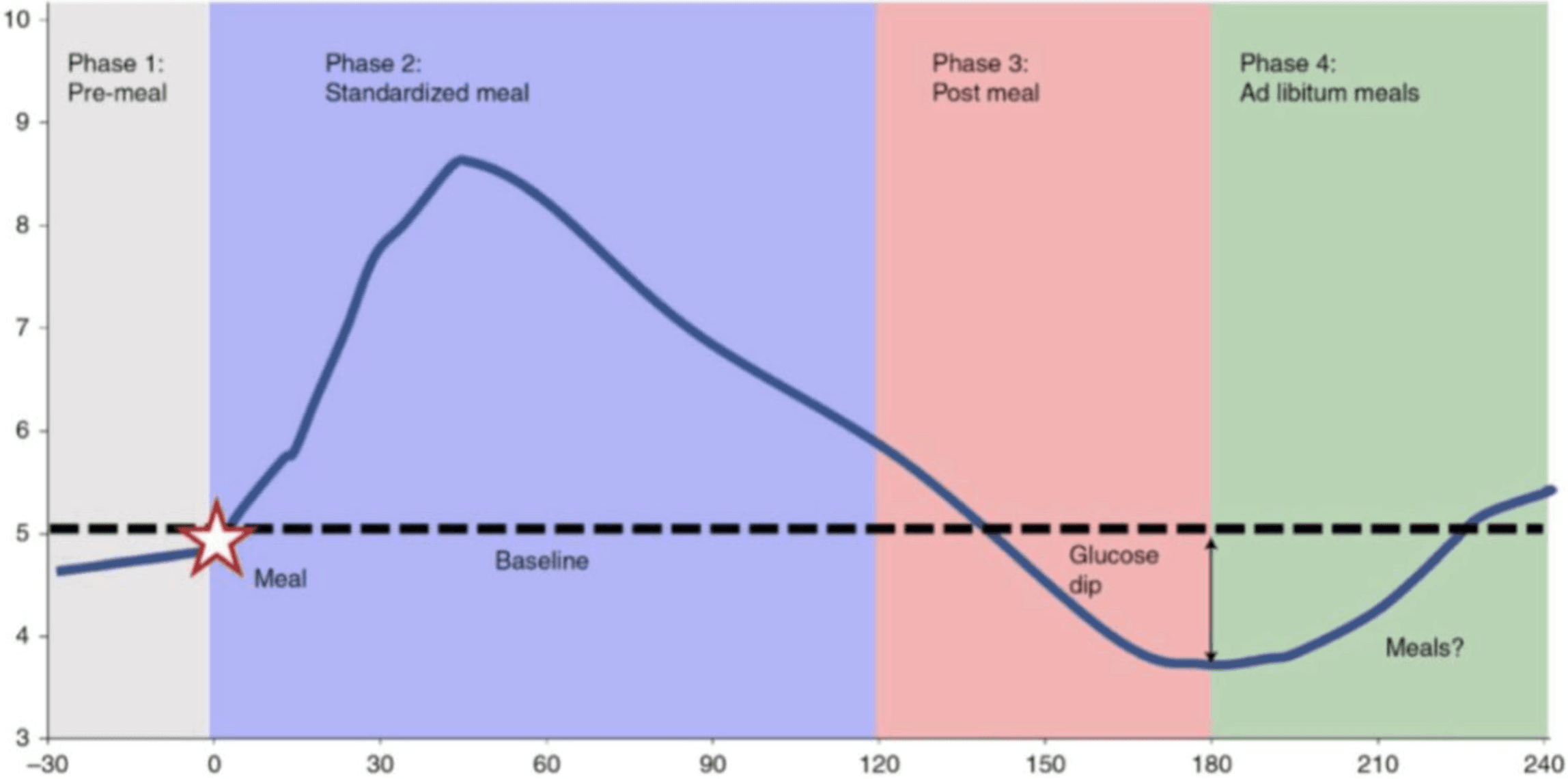
Glucose disposal depends largely on insulin sensitivity and glycogen storage capacity. Timing carbohydrate intake to coincide with periods of heightened insulin sensitivity, such as post exercise, optimizes glucose uptake and glycogen replenishment. Additional interventions, such as thermal stress in the form of contrast therapy, is further discussed in my prior article "The Physiological Menu." Conversely, consuming large amounts of carbohydrates late in the evening or before sleep may lead to higher postprandial glucose spikes and less efficient storage. Beyond the type and timing of carbohydrates, fiber content and overall meal composition significantly influence post meal glucose dynamics. High fiber, minimally processed foods slow glucose absorption and improve insulin response, while combining carbohydrates with protein and healthy fats can blunt spikes in blood sugar.
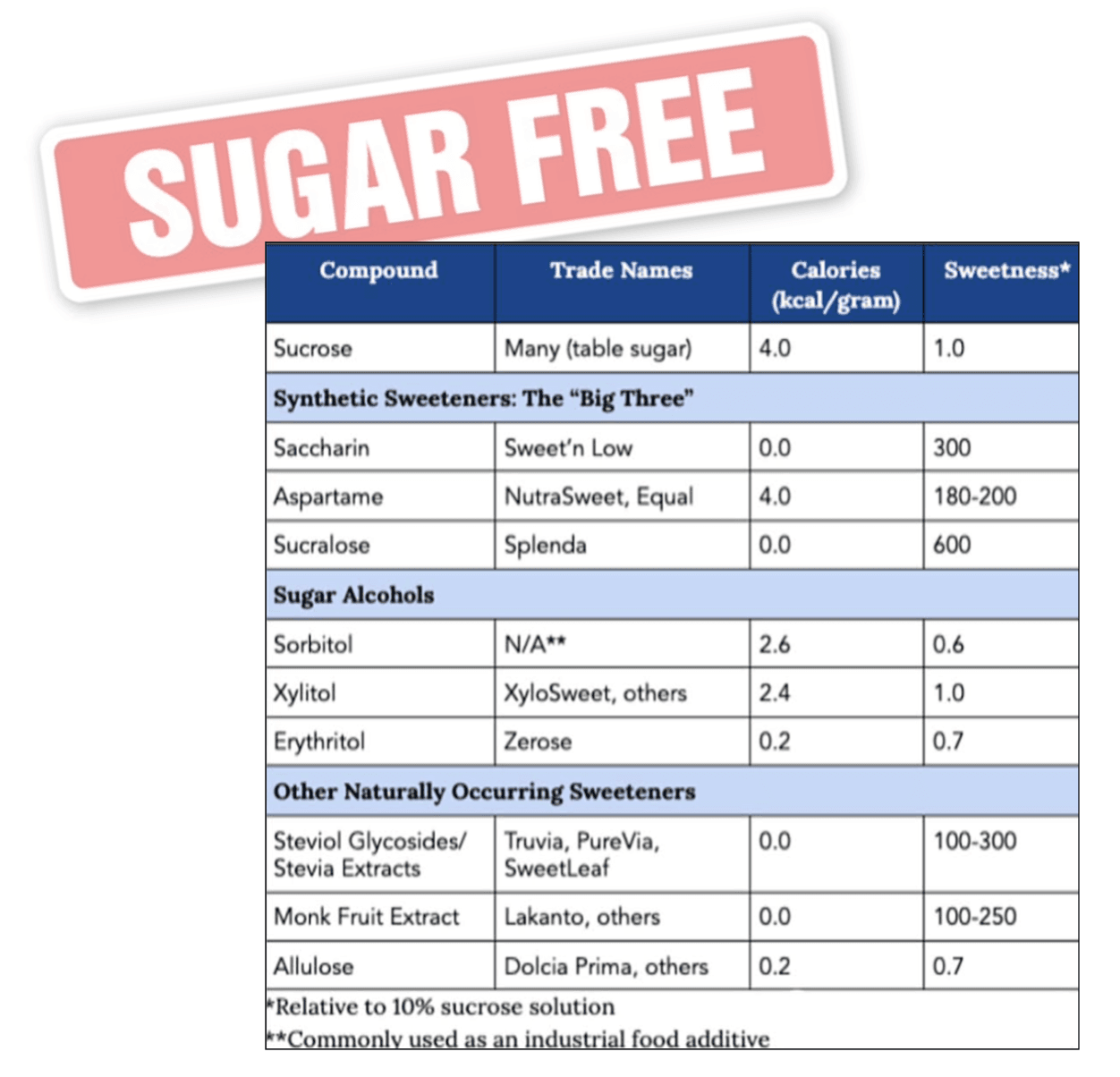
Artificial sweeteners and zero calorie sugar substitutes can also be useful tools for reducing added sugar intake and managing blood glucose. While they do not fully replicate the satiety or reward effects of natural sugars, when used strategically and combined with whole, nutrient dense foods, they can support long term adherence to a healthy diet. Monitoring hunger cues, portion sizes, and glucose responses allows individuals to leverage these alternatives effectively without undermining metabolic goals. In practice, prioritizing whole food carbohydrates, limiting added sugars, timing carbohydrate intake appropriately, and incorporating sugar substitutes when necessary creates a comprehensive nutritional strategy to support glucose regulation, reduce the risk of Type 2 diabetes and promote long term metabolic health.

The World Health Organization (WHO) recommends that adults aged 18–64 engage in 150–300 minutes of moderate intensity aerobic activity (such as brisk walking) per week, or 75–150 minutes of vigorous intensity activity (such as running or cycling), or an equivalent combination. Additionally, resistance exercise involving major muscle groups should be performed at least two days per week. These guidelines are closely aligned with recommendations from the American College of Sports Medicine (ACSM) and the Centers for Disease Control and Prevention (CDC), making them a globally recognized standard for health promotion. Understanding these baseline recommendations provides a foundation for exploring how exercise directly influences glucose regulation and insulin sensitivity. Importantly, these guidelines are grounded in decades of research demonstrating strong links between regular physical activity and reduced risk of chronic diseases, including cardiovascular disease, Type 2 diabetes, obesity, and certain cancers. As I discuss in depth in my prior article "Working In: Exercises Role In Mental Health," physical activity also supports mental health and cognitive function.

Despite these well established recommendations, adherence often fluctuates across the lifespan. In early adulthood, structured environments such as sports teams, schools or gyms can facilitate regular activity. However, as professional and family responsibilities increase, access to these supports may diminish, making consistent engagement in exercise more challenging. Recognizing these real world barriers highlights the importance of practical strategies that integrate physical activity into daily routines. Minimizing sedentary behavior is equally crucial, with replacing even brief periods of inactivity with light activity having measurable health benefits. By applying these findings to drive daily behaviors, we can see how structured, increased daily movement creates a protective metabolic environment.

In the United States, the average adult consumes roughly 3,540 kcal per day while engaging in less than 20 minutes of physical activity, according to the Food and Agriculture Organization (FAO). This mismatch between energy intake and expenditure leads to insulin resistance, weight gain and significantly increases the risk of Type 2 diabetes. As Dr. Layne Norton notes, "most people are stepping over dollars to pick up pennies," highlighting the tendency to prioritize marginal strategies over foundational habits. While supplemental tools, whether experimental, interest driven or highly nuanced, can enhance progress, their effectiveness depends on consistent behavior and lifestyle changes. This imbalance emphasizes the need for exercise strategies that complement nutrition interventions to restore metabolic health.
Skeletal muscle is the body’s largest site of glucose disposal, responsible for up to 80% of insulin-stimulated glucose uptake. This process relies on GLUT4 (Glucose Transporter Type 4), a transporter protein stored within muscle cells that acts as a molecular gate for glucose entry. In response to insulin, GLUT4 moves to the cell surface, allowing glucose to flow from the bloodstream into muscle tissue. By increasing lean muscle mass, resistance training expands this glucose handling capacity, effectively turning skeletal muscle into a powerful “glucose sink.” These adaptations enhance insulin sensitivity, reduce circulating blood glucose and provide broad metabolic benefits that are critical to preventing and managing diabetes.
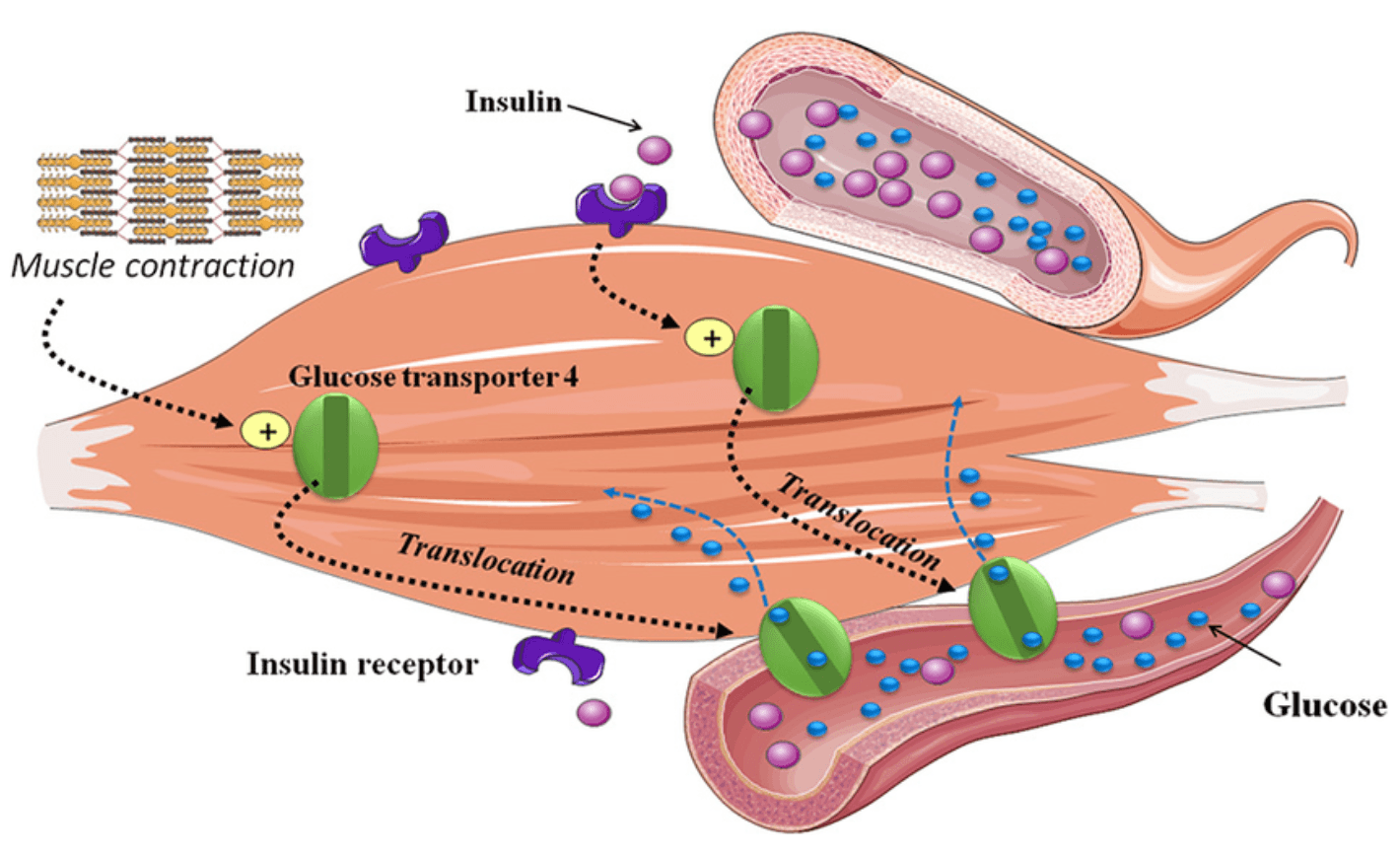
Equally important, exercise triggers a second pathway of glucose uptake that operates independently of insulin. When muscles contract, they activate AMP-activated protein kinase (AMPK), the cell’s energy sensor. Once stimulated, AMPK drives GLUT4 translocation to the cell membrane in a manner parallel, but independent to insulin. This mechanism explains how, even in states of insulin resistance, glucose can still be effectively cleared from circulation during physical activity. Fortunately, the impact is both immediate and lasting. Exercise-induced GLUT4 activity enhances glucose uptake for hours after a workout, while long term resistance training increases overall GLUT4 expression, making muscle more metabolically efficient. Together, these dual entry systems, insulin-mediated and muscular contraction driven, position skeletal muscle as the central regulator of glucose homeostasis and highlight why regular exercise is indispensable for the prevention, treatment and even reversal of metabolic disease
Several natural supplements have gained strong scientific interest for their role in supporting healthy blood glucose regulation, particularly in individuals with insulin resistance, prediabetes or Type 2 diabetes. Among the most studied are berberine, apple cider vinegar, and chromium picolinate, each targeting different aspects of glucose metabolism with varying degrees of clinical support. While not substitutes for pharmacologic therapy in advanced disease, these compounds demonstrate mechanisms that parallel or complement established treatments, offering potential benefit within a comprehensive exercise, nutrition and lifestyle plan.

Berberine, a plant-derived bioactive compound, is one of the most extensively researched natural agents. Studies consistently show that it improves insulin sensitivity, reduces hepatic glucose production and activates AMPK. These effects translate into reductions in fasting glucose, HbA1c and lipid profiles, making berberine a promising adjunct for metabolic health. Though generally well tolerated, gastrointestinal side effects may occur, and variability in product quality requires careful consideration. Apple cider vinegar (ACV) has also demonstrated glucose lowering effects, particularly when consumed before meals. By slowing gastric emptying, reducing carbohydrate absorption and activating AMPK, ACV helps blunt postprandial glucose spikes and enhance muscle glucose uptake. While each produce more modest results than pharmacologic agents, their accessibility, affordability and added benefits on satiety and digestion make them a practical dietary adjunct.
Chromium picolinate, an essential trace mineral, contributes to insulin action by enhancing receptor signaling. Supplementation can improve fasting glucose and insulin sensitivity, especially in individuals with chromium deficiency or Type 2 diabetes. Benefits appear dose-dependent, with clinically relevant effects observed at appropriate intakes. Together, these supplements offer complementary pathways for supporting blood glucose regulation. While not replacements for prescription therapies in advanced disease, they represent research supported options within a broader strategy of lifestyle, nutrition and metabolic health optimization. When applied thoughtfully, they provide additional tools for preventing disease progression and supporting glycemic stability across the continuum of metabolic dysfunction.

Effective diabetes prevention, management and reversal may require more than lifestyle strategies alone. While diet, exercise, and behavioral interventions form the foundation of metabolic health, pharmacologic therapies play a critical complementary role by targeting specific physiological mechanisms underlying hyperglycemia and insulin resistance. Understanding these medications and their mechanisms of action help understand how they support glucose control, weight management and long term health.
Targeting different aspects of glucose regulation, Acarbose (an alpha-glucosidase inhibitor) and Canagliflozin (an SGLT2 inhibitor) illustrate complementary pharmacologic strategies. Acarbose slows carbohydrate digestion in the small intestine, blunting postprandial glucose spikes and improving overall glycemic control. Canagliflozin, by contrast, blocks renal glucose reabsorption via SGLT2 transporters, promoting glucose excretion in urine, lowering blood glucose, supporting weight loss, and offering cardiovascular and renal benefits. SGLT2 inhibition typically blocks approximately 90% of renal glucose reabsorption, with variations depending on the drug and individual factors. Both approaches exemplify how targeting glucose handling, either at absorption or excretion, can effectively manage type 2 diabetes.
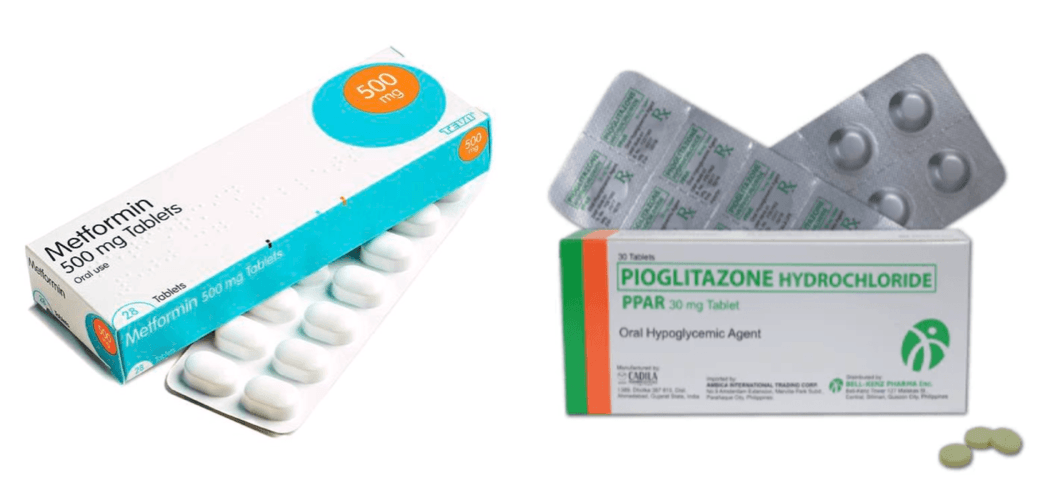
Metformin, typically first line therapy, exerts its primary effect in the liver by suppressing hepatic glucose production mediated, in part, through activation of AMPK, a central regulator of cellular energy metabolism. Beyond the liver, metformin also improves insulin sensitivity in skeletal muscle and adipose tissue and has been shown to alter gut microbiota and enhance GLP-1 secretion, further contributing to its glucose lowering effects. Its safety profile and low risk, in addition to low cost, of hypoglycemia make it a common selection for diabetes management, with additional research exploring potential benefits for longevity and cancer prevention.
Pioglitazone works primarily by activating PPAR-γ, which modifies gene expression to improve insulin sensitivity and enhance GLUT4 mediated glucose uptake in skeletal muscle, adipose tissue and the liver. This leads to increased glucose uptake, reduced hepatic glucose production, improved lipid metabolism and reduced systemic inflammation. It is well established as an effective treatment for improving glycemic control, lipid metabolism, and overall metabolic health, with particular benefit in patients with insulin resistance, metabolic syndrome or NAFLD.
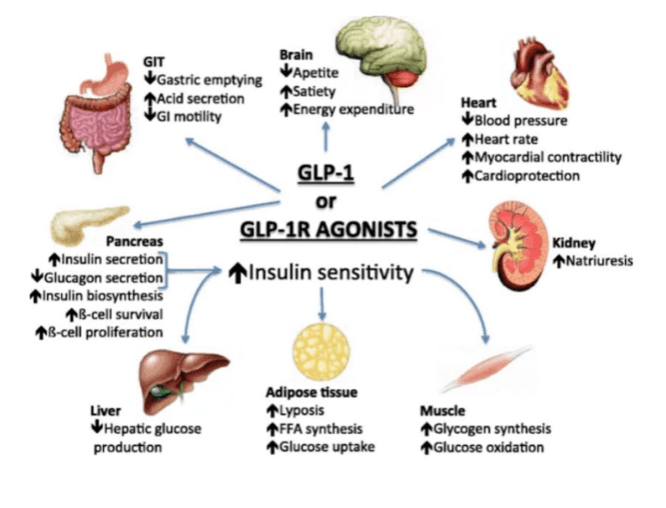
Beyond these mechanisms, gut-derived incretin hormones play a critical role in regulating insulin secretion and appetite. GLP-1 is released in response to food intake, enhancing insulin release from pancreatic beta cells, a process called the “incretin effect.” In Type 2 diabetes, this effect is diminished, contributing to impaired glucose control. GLP-1 receptor agonists (such as Ozempic) mimic this hormone, improving insulin secretion, suppressing glucagon, slowing gastric emptying and promoting satiety, collectively lowering blood glucose and supporting weight management. Current evidence shows that GLP-1 receptor agonists promote significant fat loss but can also reduce lean mass, typically accounting for about 25–40% of total weight lost. Clinical trials suggest that muscle function is often preserved despite some loss of lean tissue. For this reason, it is imperative to pair the previously mentioned lifestyle, nutrition and exercise habits with GLP-1 therapy to help maintain skeletal muscle and support long term metabolic health.
Lifestyle +
The most effective strategy for diabetes prevention, management and reversal combines early detection with targeted interventions that address the root causes of metabolic dysfunction. Identifying skeletal muscle insulin resistance before it progresses allows for timely, personalized action. Foundational lifestyle modifications including exercise, nutrition and behavioral strategies further enhance insulin sensitivity, optimize glucose regulation and reduce disease risk. Incorporating pharmacologic therapies complement these approaches by targeting specific metabolic pathways when lifestyle changes alone are insufficient. Strategic use of tools such as continuous glucose monitors provide real time, actionable feedback, enabling precise adjustments to diet, activity and medication for individualized optimization. By integrating early detection, lifestyle, pharmacology, and digital insights into a cohesive, evidence based framework, individuals can achieve the best long term outcomes for metabolic and overall health.